Complete response to pembrolizumab as a single agent in a patient with stage III NSCLC with high PD-L1 expression: a case report
Accepted: November 14, 2022
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
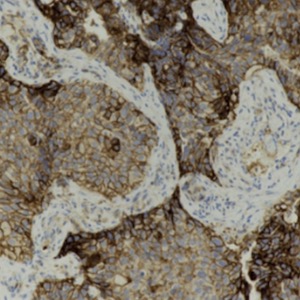
Non‐small cell lung cancer (NSCLC) accounts for 75-80% of all lung cancer cases. Stage III NSCLC represents a highly heterogenous stage characterized by different disease presentations and a wide range of treatment options. For patients with good performance status and unresectable-stage III NSCLC with programmed death-ligands 1 (PD-L1) tumor proportion score (TPS) ≥1%, durvalumab consolidation immunotherapy after a platinum-based chemo-radiotherapy is strongly recommended. However, age, poor performance status, underlying comorbidities may represent contraindications for chemotherapy to be used in a subgroup of patients. Herein, we report a case of an 80-year-old male affected by a stage IIIB lung adenocarcinoma with overexpression of PD-L1 (TPS 90%) treated with pembrolizumab, an immune checkpoint inhibitor targeting PD-1/PD-L1 pathways, which shows a complete resolution of lung lesion after four cycles of treatment. Although randomized controlled trials are required, this case report may suggest the potential role of pembrolizumab for chemotherapy unsuitable patients with overexpressing PD-L1 unresectable-stage III NSCLC.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/monaldi.2022.2440
https://doi.org/10.4081/monaldi.2022.2440




