Subcutaneous finger cardioverter-defibrillator in low weight paediatric patients: a case series
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
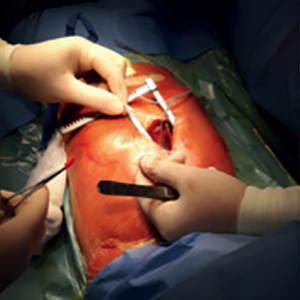
Placement of traditional transvenous implantable cardioverter defibrillator (ICD) system in low-weight children is often difficult because of their vessel size, the elevated risk of lead malfunction and failure, children’s growth and various anatomic constraints, creating the need for alternative solutions. Subcutaneous array leads combined with an abdominally placed ICD device can minimize the surgical approach. In this case series, we analyse the data behind indications for subcutaneous finger cardioverter defibrillator (SFCD) and discuss the preliminary clinical experience in low-weight children. We considered 4 consecutive children (mean age 3.9 years, range 3-5.5 years, mean body weight 17.6 Kg, range 14-23 Kg) who underwent SFCD implant from April 2016 to August 2020. All patients showed a good compliance to the device system with no complications (infections or skin erosions). No patients experienced in the observation period (mean time 44.5±21.5 months) sustained ventricular arrhythmias requiring shocks. No inappropriate shocks released by the device occurred. No significant changes were observed in LET (lowest energy tested) performed around 24 months of follow-up. All patients showed a good compliance and stable atrio-ventricular sensing and pacing thresholds. In smaller children in whom a transvenous approach is not feasible or not possible for anatomic reasons, the SFCD appears to be a safe method to prevent SCD with little surgical trauma and preservation of an intact vascular system, providing an adequate bridge to transvenous ICD or subcutaneous ICD implant late in the life.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






