Pulmonary adverse events due to immune checkpoint inhibitors: A literature review
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
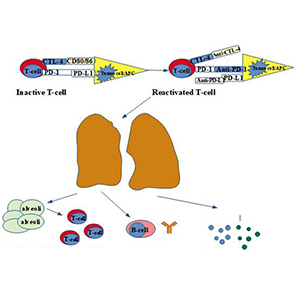
Cancer immunotherapy aims to stimulate the immune system to fight against tumors, utilizing the presentation of molecules on the surface of the malignant cells that can be recognized by the antibodies of the immune system. Immune checkpoint inhibitors, a type of cancer immunotherapy, are broadly used in different types of cancer, improving patients’ survival and quality of life. However, treatment with these agents causes immune-related toxicities affecting many organs. The most frequent pulmonary adverse event is pneumonitis representing a non-infective inflammation localized to the interstitium and alveoli. Other lung toxicities include airway disease, pulmonary vasculitis, sarcoid-like reactions, infections, pleural effusions, pulmonary nodules, diaphragm myositis and allergic bronchopulmonary aspergillosis. This review aims to summarize these pulmonary adverse events, underlining the significance of an optimal expeditious diagnosis and management.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






