Right-sided infective endocarditis and pulmonary embolism: a multicenter study
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
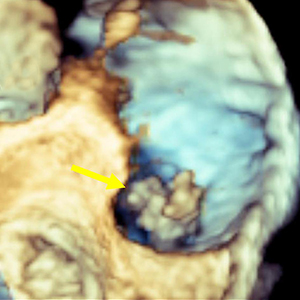
The incidence of right-sided infective endocarditis (RSIE) is steadily increasing and it has been reported to be associated with high risk of embolic events (EE). Aim of our study was to identify the clinical characteristics of patients with RSIE complicated by PE. Indeed, the identification of patients at high risk of significant PE who will benefit from a more aggressive therapeutic strategy may improve the prognosis. From January 2015 to September 2020, 176 patients (Pts) in 6 centers were found to have definite RSIE complicated by PE. Advanced imaging for PE including computed tomography pulmonary angiography (CTPA) was performed in 28 pts (16%) who represent our study group (24 male, mean age 50.6 ±18.29 years). They all underwent transesophageal echocardiography (TEE), in 12 cases (43%) also three-dimensional (3D) TEE, and 27 patients (99%) had both TEE and transthoracic echocardiography (TTE). A total of 53 vegetations (V) were detected. In 18 pts (64%) two or more vegetations were found. Native tricuspid valve was the most frequently involved valve (38 V, 71.7%), followed by catheter (5 V, 9.4%), tricuspid valve prosthesis (4 V, 7.5%), chordae and papillary muscle (2 V, 3.8%) and one vegetation (9%) in each of the following: pulmonic valve, inferior vena cava, eustachian valve, and right atrium. The most common location for vegetations was the anterior leaflet of the tricuspid valve (19 V, 35.8 %) followed by the posterior leaflet (11 V, 20.8%). The most common vegetations morphology was raceme-like shaped (35.8%). Staphylococcus aureus (S. aureus) was the most common causative pathogen (14 pts, 50%). The incidence of PE was very high in patients with vegetation length above 1.5 cm (median 17.6±6.5 mm by TEE). Our results suggest that a routine CTPA should be advised in the presence of vegetations larger than 1.5 cm and with S. aureus infection. This behavior would identify patients at high risk of PE who will benefit from a more aggressive therapeutic strategy, leading to an improvement in the prognosis. Further prospective studies are required to better confirm our hypothesis.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






